Resource Carbon Dioxide
Utilization of CO2 streams to broaden the raw material base of chemical products
Utilization of CO2 streams to broaden the raw material base of chemical products
Resource Carbon Dioxide
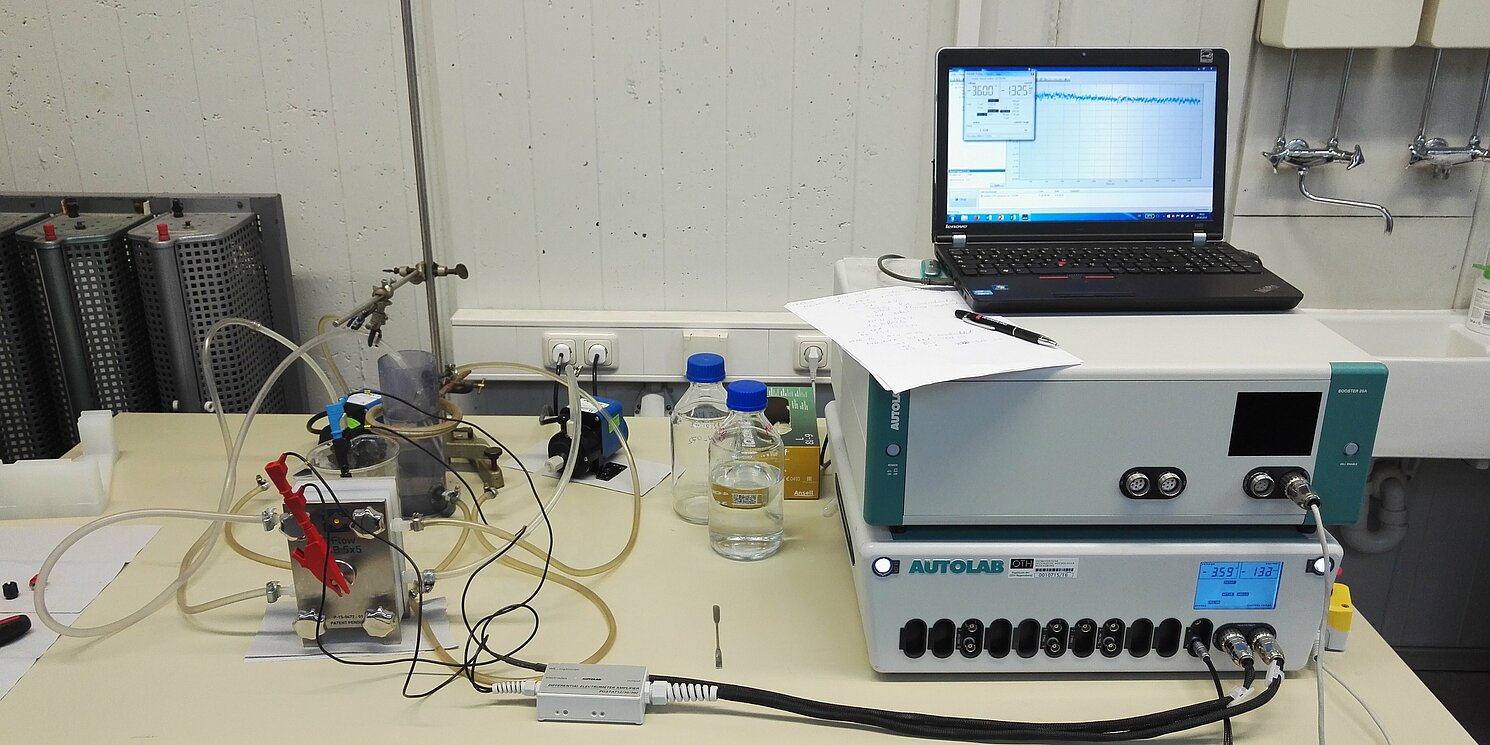
As part of the Bavarian Research Foundation-funded project "Resource carbon dioxide: Utilization of CO2 streams to broaden the raw material base of chemical products", silver-based catalysts for the electrochemical carbon dioxide CO2 reduction to carbon monoxide CO were produced and tested for their electrochemical properties in collaboration with RAS AG.
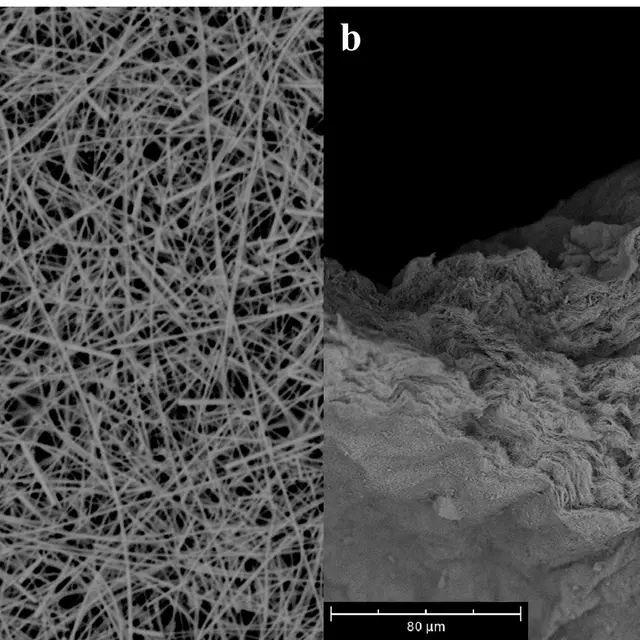
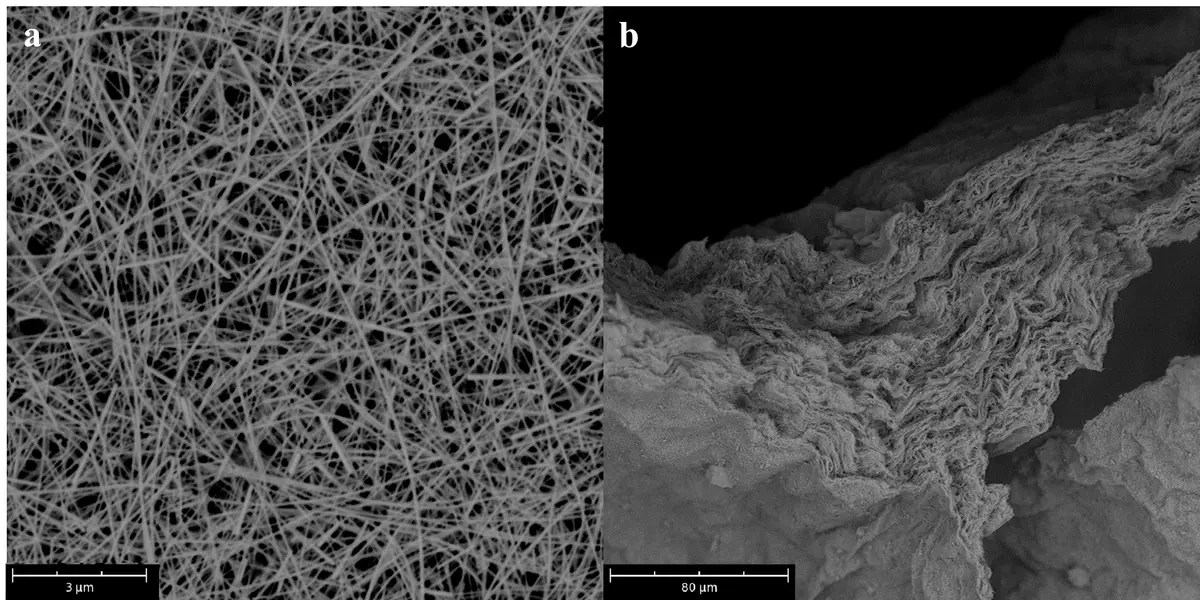
For this purpose, silver nanowires (diameter 30-50 nm, length 10-40 μm) were successfully synthesized and used as a starting material for catalyst production. Non-woven catalysts were produced from this silver nanowire dispersion and prepared for use in test electrolysis cells or in a realistic cell. The top view of the surface of a catalyst (a) and the cross-section (b) of a sample are shown below.
The catalysts were first tested for their electrochemical properties in an electrochemical half-cell with a potentiostat. Impedance measurements, cyclic voltammetry measurements and linear sweep voltammetry measurements were carried out to estimate the performance. The mechanical stability of the catalysts was tested in a simple flow cell.
For measurements under more realistic conditions, a test electrolysis cell (C-Flow LAB Cell) was purchased in which the catalyst was integrated as a cathode. Here, electrochemical measurements could be carried out under more realistic conditions to estimate performance and determine long-term stability. A maximum current density of -215.6 mA/cm2 at a cell voltage of -3.1 V was achieved in this cell. In relation to the amount of silver (Ag) used, a material-specific current density of -21.5 A/g(Ag) was achieved. A long-term stability of the catalysts of > 100 hours was easily achieved by carrying out chronoamperometry measurements. The load cycle stability was also confirmed in the course of the long-term stability measurements.
Gas analysis measurements were carried out in the same cell setup. Infrared spectroscopy was used to qualitatively determine the product gas and detect CO. By using special tubes from Dräger to determine the CO concentration, it was possible to estimate the amount of CO produced in the product gas. However, only a low selectivity of 5 % to 28 % was measured at various voltages.